(a) Alkali metals dissolve in liquid ammonia to give deep blue solution due to formation of ammoniated electron which absorbs energy in the visible region of light.
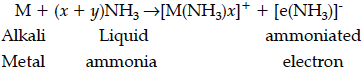
(b) Beryllium and magnesium do not give colour to the flame whereas other alkaline earth metals do so because the electrons in beryllium and magnesium are too strongly bound to get excited by flame.
(c) Potassium carbonate cannot be prepared by Solvay process because potassium hydrogen carbonate so formed is too soluble to be precipitated by the addition of ammonium hydrogen carbonate to a saturated solution of potassium chloride.