Hydrolysis of water:
At anode: 2H2O → 4H+ + O2 + 4e-……..(1)
At cathode: 2H2O + 2e- → H2 + 2 OH-
Overall reaction: 6H2O → 4H- + 4OH- +2H2 + O2 (or)
Equation (1) + (2) x 2
= 2H2O → 2H2 + O2
According to Faraday’* Law of electrolysis, to electrolyse two mole of Water (36g ≃ 36 mL. of H2O), 4F charge is required alternatively, when 36 mL of water is electrolysed, the charge generated = 4 x 96500 C.
When the whole water which is available on the lake is completely electrolysed the amount of charge generated is equal to
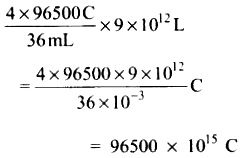
Given that in 1 second, 2 x 106 C is generated therefore, the time required to generate
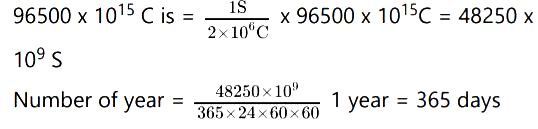
= 1.5299 x 106
= 365 x 24 hours
= 365 x 24 x 60 min
= 365 x 24 x 60 x 60 sec