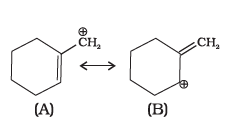
Answer the questions on the basis of information given below:
“Stability of carbocations depends upon the electron releasing inductive effect of groups adjacent to positively charged carbon atom involvement of neighbouring groups in hyperconjugation and resonance.”
Which of the following ions is more stable? Use resonance to explain your answer.