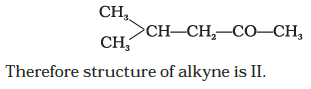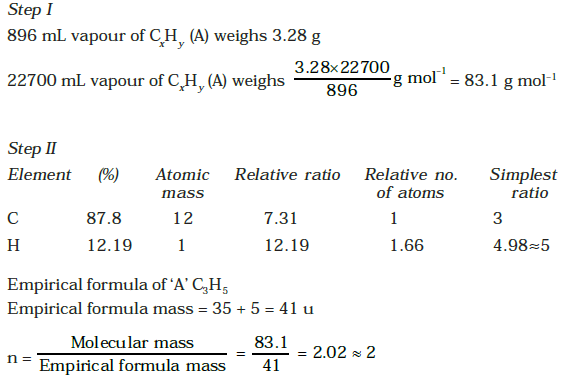
⇒ Molecular mass is double of the empirical formula mass.
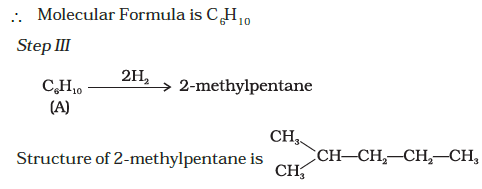
Hence, the molecule has a five carbon chain with a methyl group at the second carbon atom.
‘A’ adds a molecule of H2O in the presence of Hg2+ and H+, it should be an alkyne. Two possible structures for ‘A’ are :
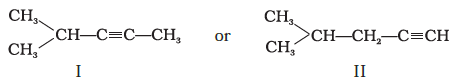
Since the ketone (B) gives a positive iodoform test, it should contain a —COCH3 group. Hence the structure of ketone is as follows :