(a) (i) Sodium – 2, 8, 1 (ii) Chlorine – 2, 8, 7
(b) (i) Sodium = 1 (ii) Chlorine = 7
(c)
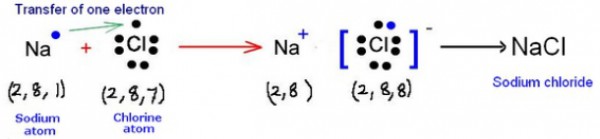
(d) Sodium chloride has a high melting point because it is an ionic compound and these compounds are made of up of positive and negative ions. There is a strong force of attraction between the oppositely charged ions, so, a lot of heat energy is required to break this force of attraction and melt or boil the ionic compound.
(e) Anode: Thick block of impure copper metal; Cathode: Thin strip of p ure copper metal