Solids
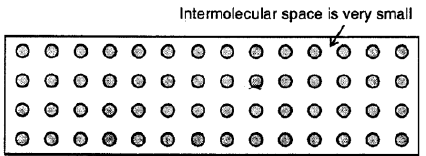
Here the molecules are very tightly packed that there is no or very less intermolecular space and there is high intermolecular force of attraction (force of cohesion). The molecules do not move about their mean position and thus solids have a definite shape and volume.
Liquids :
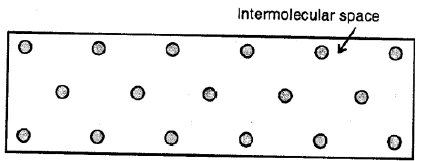
Here the molecules are less tightly packed as compared to solids and also there is a lesser force of intermolecular attraction. The intermolecular distance is greater than that in the solids. Thus, they do not have a definite shape but acquire the shape of the vessel in which they are contained but have a definite volume at a given temperature.
Gases :
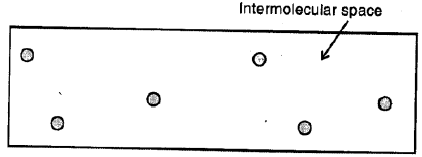
Here the molecules are far apart from each other i.e. have the greatest intermolecular distance which results in the weakest intermolecular forces of attraction. The molecules as are not bound by any strong force, move about freely and thus gases do not have a definite shape and also do not have any definite volume.