(a) When Zinc reacts with the dilute sulphuric acid, hydrogen gas is evolved with an effervescence.
Zn + dil. H2SO4 → Zn SO4 + H2.
(b) When a few pieces of iron are dropped into a blue ** copper sulphate solution, the blue **** of the solution fades and eventually turns to green.
(c) When a solution of silver nitrate is added to a solution of sodium chloride, white insoluble ppt. of silver chloride is formed.
AgNO3 (aq) + NaCl (aq) → AgCl (ppt) + NaNO3(aq)
(d) When ferrous sulphate solution is added to sodium hydroxide solution, a ***** green ppt. of ferrous hydroxide is formed.
FeSO4 (aq) + 2NaOH (aq) → Fe(OH)2 ↓ + Na2SO4(aq)
(e) When solid lead nitrate is heated, it decomposes to produce light yellow solid lead monoxide, reddish brown nitrogen dioxide gas and colorless oxygen gas.
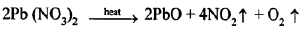
(f) When few drops of dilute sulphuric acid are added to barium chloride solution, a white precipitate of barium sulphate is formed.
