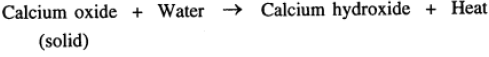(a) ***** solid mass (charcoal) is formed along with water vapours.
(b) Manganese dioxide acts as a catalyst for the decomposition of potassium chlorate into potassium chloride and oxygen at a lower temperature.
(c) Sodium acetate, CO2 and water are formed.
(d) A white insoluble solid precipitate of silver chloride is formed along with Sodium nitrate.
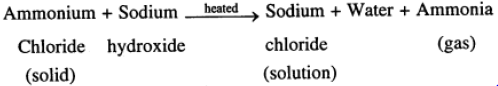
(e) When solid ammonium chloride is heated with sodium hydroxide solution, a gas ammonia is evolved which is recognised by its strong pungent smell.
(f) When water is added to quick lime, a large amount of heat energy is evolved.