(a) Power of the medium wave transmitter, P = 10 kW = 104 W = 104 J/*
Hence, energy emitted by the transmitter per second, E = 104
Wavelength of the radio wave, λ = 500 m
The energy of the wave is given as:
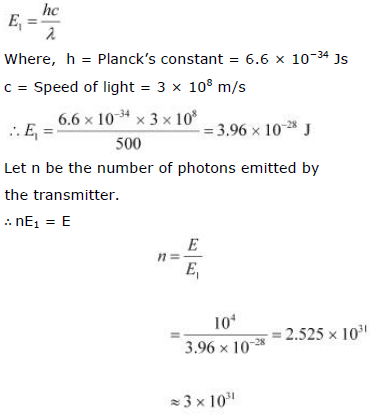
The energy (E1) of a radio photon is very less, but the number of photons (n) emitted per second in a radio wave is very large.
The existence of a minimum quantum of energy can be ignored and the total energy of a radio wave can be treated as being continuous.
(b) Intensity of light perceived by the human eye, I = 10−10 W m−2
Area of a pupil, A = 0.4 cm2 = 0.4 × 10−4 m2
Frequency of white light, ν= 6 × 1014 Hz
The energy emitted by a photon is given as: E = hν
Where,
h = Planck’* constant = 6.6 × 10−34 Js
E = 6.6 × 10−34 × 6 × 1014
= 3.96 × 10−19 J
Let n be the total number of photons falling per second, per unit area of the pupil. The total energy per unit for n falling photons is given as:
E = n × 3.96 × 10−19 J s−1 m−2
The energy per unit area per second is the intensity of light.
E = I
n × 3.96 × 10−19 = 10−10
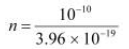
= 2.52 × 108 m2 s−1
The total number of photons entering the pupil per second is given as:
nA = n × A
= 2.52 × 108 × 0.4 × 10−4
= 1.008 × 104 s−1
This number is not as large as the one found in problem (a), but it is large enough for the human eye to never see the individual photons.