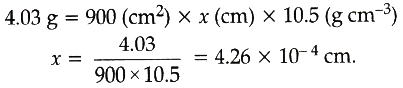Calculation of mass of Ag deposited :
The electrode reaction is Ag+ + e- → Ag
The quantity of electricity passed
= Current x Time
= 0.5 (amp.) x 2 x 60 x 60 (sec)
= 3600 C.
From the electrode reaction, it is clear that 96500 C of electricity *** Ag = 108 g
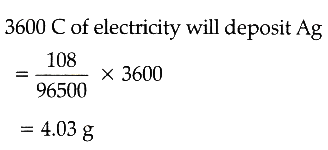
Calculation of thickness:
Let the thickness of silver deposited be x cm.
Mass = Volume x Density
= Area x Thickness x Density
(Volume = Area x thickness)