The gradual decrease in atomic and ionic size of lanthanoids with increase in atomic number is known as lanthanoid contraction.
Cause of lanthanoid contraction :As the atomic number increases, the positive charge on nuclear increases by +1 unit and one more electron enters in the same 4f subshell. There is imperfect shielding of one electron by another electron in the same 4f subshell. The extent of shielding for electrons is less in 4f subshell as compared to electrons in d subshell. Hence, with increase of nuclear charge the valence shell is pulled slightly towards nucleus. Due to this pull, the size of M3+ ions go on decreasing with increasing atomic number.
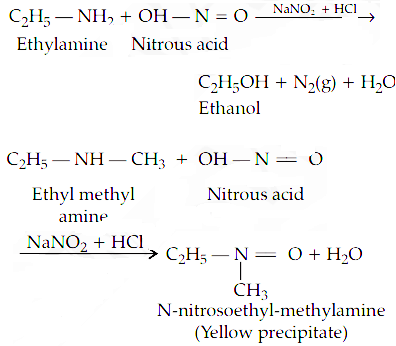
Structure of chloroxylenol
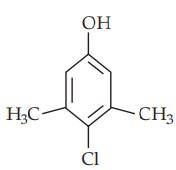
Structure of adenine
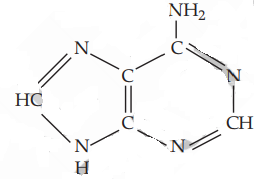
Test to distinguish between ethylamine and ethyl methyl amine :