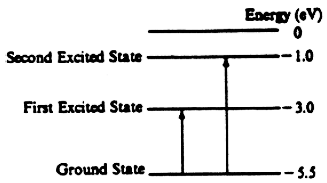
(a) E = hf … 5.5 = (4.14x10–15 eV-*) f … f = 1.33x1015 Hz
(b) i. From the –1eV state, the following transitions could happen –1ev → –3eV → –5.5 →, or –1ev → –5.5eV, three different possible energy differences: 2eV, 2.5 eV and 4.5 eV Using E = hc / λ … with hc = 1240 eV-nm, and the energies above give the following wavelengths λ1 = 621 nm, λ2 = 497 nm λ1 = 276 nm
ii. The visible range is 400–700 nm, so the 1st and 2nd wavelengths are in that range.
(c) The energies corresponding to the given wavelength are found with E = hc / λ and are 1.24–4.97 eV. This range allows a transition to the first and second excited state but does not allow ionization because its not enough energy for ionization.