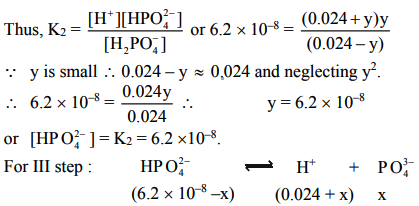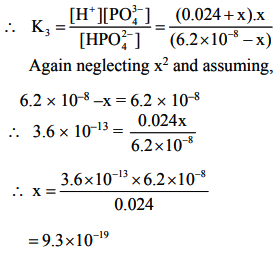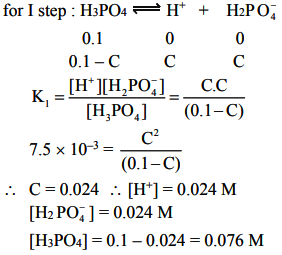
The value of K1 is much large than K2 and K3. Also dissociation of II and III steps occurs in presence of H+ furnished in I step and thus, dissociation of II and III steps is further suppressed due to common ion effect.
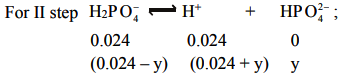
The dissociation of H2PO-4 occurs in presence of [H+] furnished in step I.