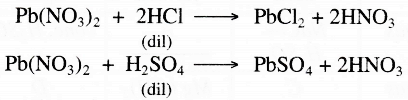
On adding lead nitrate to both acids, we will get a white precipitate. On heating the solution. the one whose precipitate will redissolve will be dil. HCI and the one with insoluble precipitate will be dil. H2SO4.
Actually on adding lead nitrate to HCI. PbCl2 precipitates out and on heating the solution it redissolves. But in case of H2SO4, PbSO4 is formed which is insoluble even on heating it.