*Kohlrausch’ law :–**
According to this law, “molar conductivity of an electrolyte at infinite dilution is expressed as sum of the contributions from its individual ions.” These contributions are called ionic conductances of cations and anion.
If λ°+ and λ°- are ionic conductances of cation and anion, then
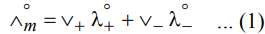
Where V+ and V- represents number of cations and number of anions.
(b) (i) Calculation of molar conductivity of weak electrolyte
Suppose we have to determine ∧°m(CH3COOH). For this, limiting molar conductivities of strong electrolytes like CH3COONa, HCl and HCl are determined.
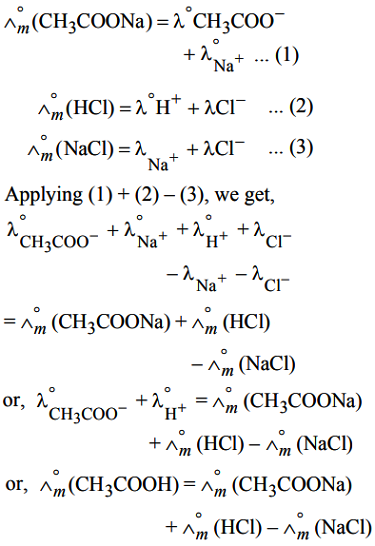
(ii) Calculation of degree of dissociation of weak electrolyte
By the knowledge of limiting molar conductivity of electrolyte, degree of dissociation of weak electrolyte is calculated by the formula–
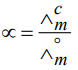
Where ∧cm is the molar conductivity of solution at any concentration.
∧°mis the limiting molar conductivity.