In carbon dioxide molecule, the two oxygen atoms are bonded on either side with carbon atom by
double bonds. Thus, there are 2 double bonds in CO2.
Carbon shares its two electrons in the formation of a double bond with one oxygen atom and another two electrons with another oxygen atom. In this process, both the oxygen atoms and the carbon atom acquire the stable electronic configuration of the noble gas neon. The formation of CO2 molecule is shown below:
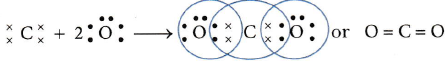
Valencies of C and O are 4 and 2 respectively.u200b