(i) When sulphur is bumtin a deflagerating spoon, it melts to form a reddish brown liquid which catches ****. It burns with a blue flame forming an extremely pungent gas sulphur dioxide.
(ii) In the laboratory, hydrogen sulphide gas is prepared by the action of dilute sulphuric acid on ferrous sulphide
FeS + H2SO4 ⟶ FeSO4 + H2S
(iii) Concentrated sulphuric acid, when heated with oxalic acid crystals, it absorbs water from oxalic acid and-mixtures of carbon monoxide and carbon dioxide is formed.
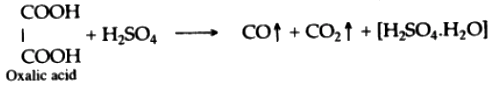
(iv) In this reaction sulphuric acid acts as a dehydrating agent.
2NaOH + H2SO4 ⟶ Na2SO4 + 2H2O
In this reaction sulphuric acid acts as an acid as it neutralizes sodium hydroxide to form salt and water only.
(v) When hydrogen sulphide gas is passed through concentrated sulphuric acid, it is oxidized to free sulphur. Sulphur dioxide and water are also formed.
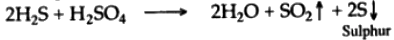
In this reaction sulphuric acid acts as an oxidizing agent