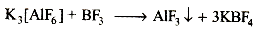AlF3 is soluble in HF in the presence of KF due to the formation of [AlF6]3- which is not formed in less dissociated anhydrous HF (stabilised due to intermolecular H bonding). The reaction looks like
3KF + AlF3 → K3[AlF6]
On adding BF3 (being more acidic than AlF3), it breaks [AIF6]3- by pulling out F- from it and forming BF-4.