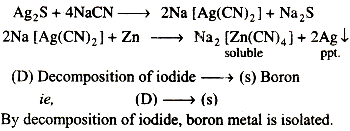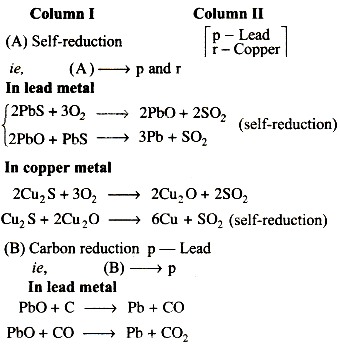
Carbon reduction process is used for the reduction of oxides of electropositive metals like Fe, Pb etc. This reduction is carried out with coal or coke on strongly heating.
(C) Complex formation and displacement by metal.
ie, (c) → q (silver)
In silver metal
Argentite mineral of Ag(Ag2S) is treated with 0.7% NaCN solution in presence of air, therefore firstly sodium argentocyanide complex is formed which on heating with zinc metal, therefore by displacement silver is isolated.