(i) Carbon monoxide (CO) is the actual reducing agent of haematite in blast furnace.
(ii) Recovery of Pb from galena :
Galena is converted to a mixture of PbO and PbSO4 when roasted at moderate temperature in presence of excess of air
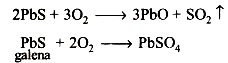
Air is cut and more galena is added. The temperature is also raised. Now, the formation of lead takes place as follows :*
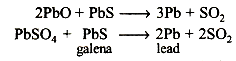
(iii) Sodium chloride prevents hydrolysis of magnesium chloride. Also, it increases the conductivity of molten magnesium chloride.
(iv) Zinc is more reducing than copper. Also, zinc is cheaper than copper. Hence, zinc (not copper) is used for the recovery of metallic silver from complex [Ag(CN)2]-.
(v) Chalcocite being a sulphide are of copper needs roasting (heating in excess of air) and not calcination.