pKb of aniline is more than that of methylamine:
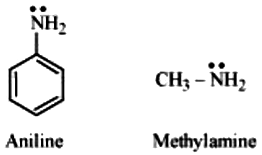
Aniline undergoes resonance and as a result, the electrons on the N-atom are delocalized over the benzene ring. Therefore, the electrons on the N-atom are less available to donate.
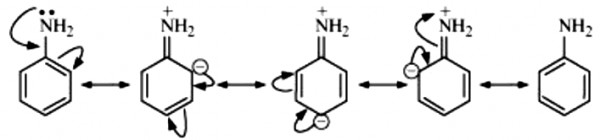
On the other hand, in case of methylamine (due to the +I effect of methyl group), the electron density on the N-atom is increased. As a result, aniline is less basic than methylamine. Thus, pKb of aniline is more than that of methylamine.
(ii) Ethylamine is soluble in water whereas aniline is not: Ethylamine when added to water forms intermolecular H−bonds with water. Hence, it is soluble in water.
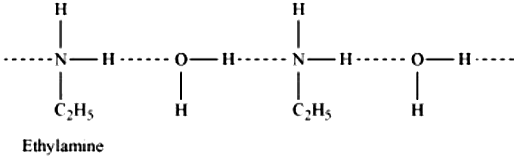
But aniline does not undergo H−bonding with water to a very large extent due to the presence of a large hydrophobic −C6H5 group. Hence, aniline is insoluble in water.