The phenoxide ion has non equivalent resonance structures in which –ve charge is at less electronegative C atom and +ve charge as at more electronegative O-atom.
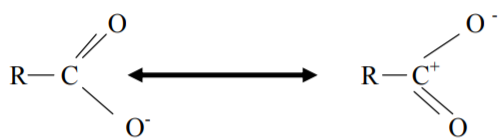
In carboxylate ion –ve charge is delocalized on two electronegative O-atoms hence resonance is more effective and a stronger acid.