i. Compound (A) is insoluble in water and burns with a smoky flame; hence it should be an aromatic compound.
ii. It has no specific element such as N, * and halogens and gives a CO2 gas with NaHCO3 solution; hence it should contain (–COOH) group.
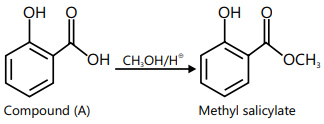
iii. It gives the oil of wintergreen (methyl salicylate) with CH3 OH in acidic medium; hence it is salicylic acid.
iv. Compound (B) is water soluble and burns with a non-smoky flame; hence it should be an aliphatic compound.
v. This compound has less carbon content because its sodium extract is prepared with sucrose and it gives a Prussian blue colour with the freshly prepared solution of FeSO4 + 2-3 drops of NaOH and a few drops of H2 SO4 ; hence it is a nitrogen-containing compound.
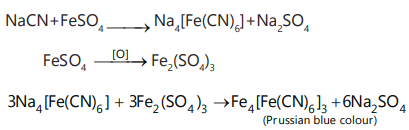
vi. On heating, it gives ammonia gas, which turns red litmus blue. Hence, it contains (-CONH2 ) group. vii. It also gives the biuret test.
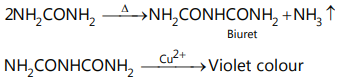
Hence, this compound is urea (NH2 CONH2 ).