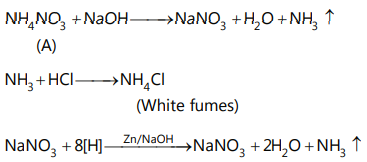
We all know that NH3 with HCl gives white fumes of NH4 Cl with popping noise.
Hence it should be NH3. Thus, compound (A) must be an ammonium salt.
Also we know that nitrates and nitrites on heating with Zn and alkali liberate NH3 gas. Hence the compound (A) should be ammonium nitrate or nitrite
But compound (A) does not give N2 on heating hence it may not be ammonium nitrite.