Correct option (d) Sulphide of all ions
Explanation:
As3+ and Cd2+ are the **** of group II, whereas Ni2+ & Zn2+ are the **** of group IV. The solubility product of group IV **** is higher as compared to group II. NH4OH increases the ionisation of H2S by removing H+ of H2S as unionisable water.
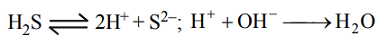
Thus excess of sulphide ions are present which leads to the precipitation of all the four ions.
Note : HCl decreases ionisation of H2S whereas NH4OH increases the ionisation of H2S.