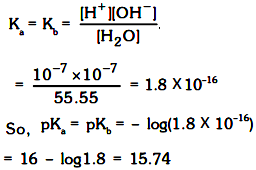Molarity = No. of *****/litre
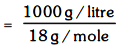
= 55.55 mole/litre = 55.55 M (density = 1 g/cc)
Ionic product of water :
According to arrhenius concept
H2O ⇌ H+ + OH– so, ionic product of water, kw = [H+][OH–] = 10–14 at 25° (exp.)
Dissociation of water is endothermic, so on increasing temperature Keq increases. Kw increases with increase in temperature.
Now pH = –log[H+] = 7 and pOH = –log[OH– ] = 7 for water at 25°C (experimental)
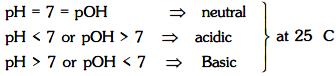
Ionic product of water is always a constant whatever has been dissolved in water since its an equilibrium constant so will be dependent only on temperature.
Degree of dissociation of water :
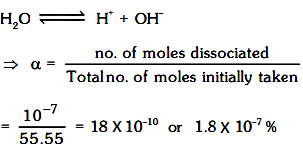
Absolute dissociation constant of water :
H2O ⇌ H++ OH–