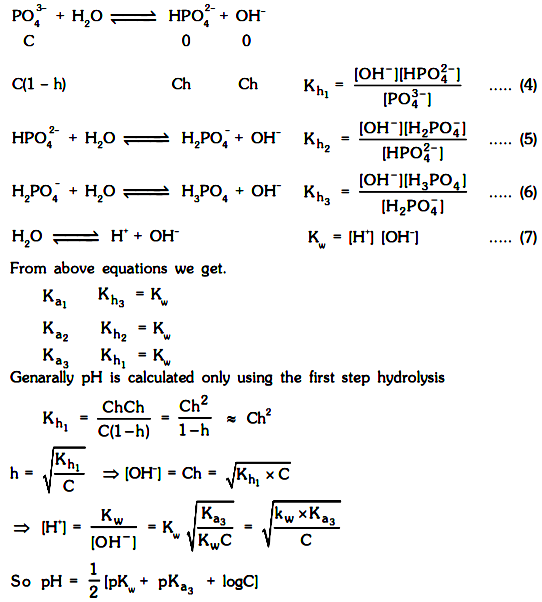
The hydrolysis of these species will take place in steps (just like dissociation of weak acids).
Out of different steps generally first step hydrolysis dominants mainly because of two reasons.
The hydrolysis constant of second and further steps is generally negligible in comparison to first step hydrolysis constant.
The second and further step hydrolysis will be suppressed in presence of ions produced due to first step hydrolysis.
For a polyprotic acid (H2S, H3PO4, H2CO3, H2C2O4) we already know that the dissociation always takes place in steps, so for example for H3PO4
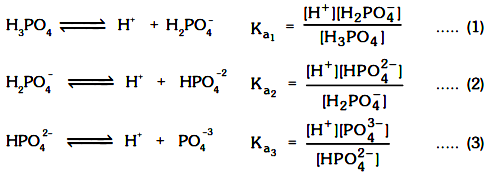
For all acids we always have Ka1 >> Ka2 >> Ka3
pH of the solution can be calculated from Ist step only because [H+ ] from IInd & IIIrd step can be neglected as
(a) Ka1 >> Ka2 >> Ka3
(b) [H+ ] from Ist dissociation will suppress the dissociation of IInd & IIIrd step. Now for the hydrolysis of polyvalent ions of salts (like K3PO4, Na2C2O4, ZnSO4, FeCl3, (NH4)2C2O4 or ions like PO43–, C2O42–, Zn2+, Fe3+ etc).
Consider the hydrolysis in step