Using Henderson'* expression
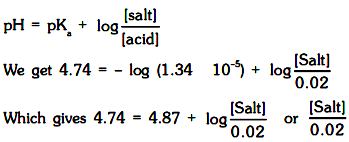
= 0.74 or [Salt] = 1.48 × 10–2 M
Hence, amount of sodium propanoate to be added = 1.48 × 10–2 × 96 g = 1.42 g
The addition of 0.01 mol of HCl converts the equivalent amount of sodium propanoate into propanoic acid. Hence, we will have
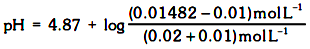
pH = 4.87 + log (0.160) = 4.87 – 0.79 = 4.08
(The pH of 0.01 molar HCl solution would be pH = – log (0.01) = 2)