The temperature of a body is the measure of the average kinetic energy of a body.
We should note that temperature of a body always depends upon its average kinetic energy and since the average kinetic energy can have a minimum possible value of zero, therefore an object cannot be cooled below a certain minimum value, this value is known as absolute zero. This is the lowest possible temperature in our universe and no object could be cooled to this temperature, it is equivalent to -273 degree Celsius or 0 Kelvin. These two scales of temperature can be converted with the following expression,
Temperature in Kelvin = Temperature in degree Celsius + 273
kinetic interpretation of temperature
- Consider one mole of a gas. Let P, V, T and M be the pressure, volume, temperature and molecular mass of the gas respectively.
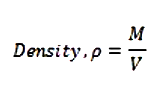
- Acc. To Kinetic theory, the pressure exerted by the gas is
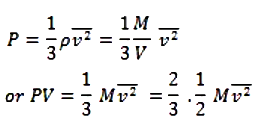
but is the average Kinetic Energy E of one mole of the gas.
PV = 2/3 E
- The ideal gas equation for one mole of a gas is PV =RT
2/3 E = RT or E = 3/2 RT
The above eq. gives the mean K.E of one mole of the gas.
- *If N is the Avogadro’ number, then the mean K.E per molecule is given by**
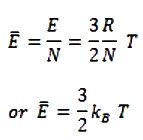
Where kB = R/N is the gas constant per molecule and is called Boltzmann’* constant.
- Thus the mean kinetic energy per molecule is proportional to the absolute temperature of the gas. It is independent of the pressure, volume and the nature of the ideal gas. Clearly,
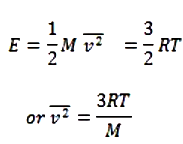
- The square root of is known as root mean square velocity and is given by
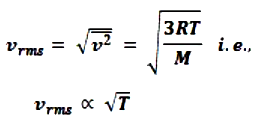
- At T = 0, vrms = 0. So, we can define absolute zero as that temperature at which all molecular motion stops.