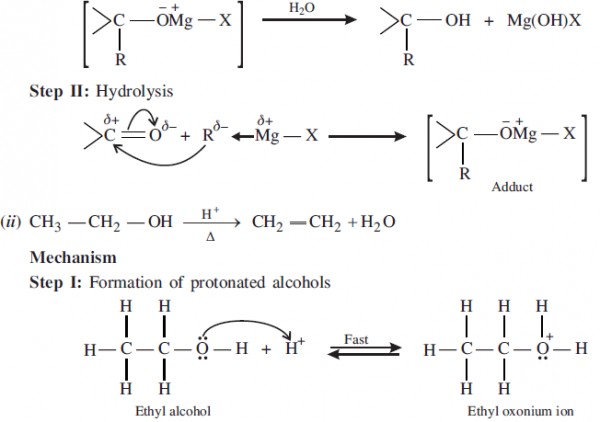
(i) Step I: Nucleophilic addition of Grignard reagent to Carbonyl group.
Step II: Formation of carbocation: It is the slowest step and hence, the rate determining step.
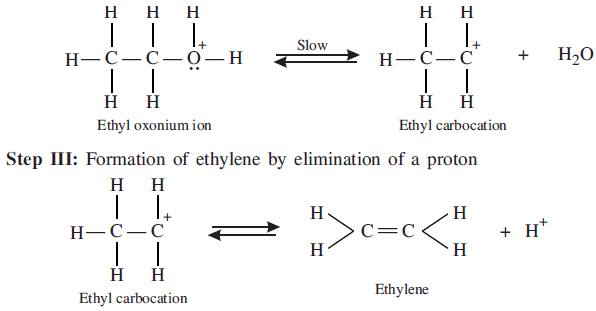
To drive the equilibrium to the right, ethylene is removed as it is formed.
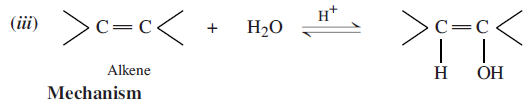
Mechanism
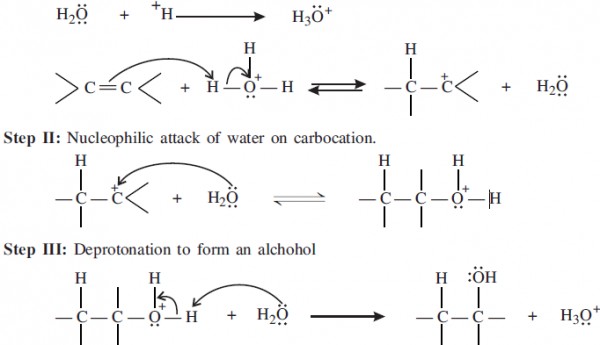
Step I: Protonation of alkene to form carbocation by electrophilic ** of H3O+.