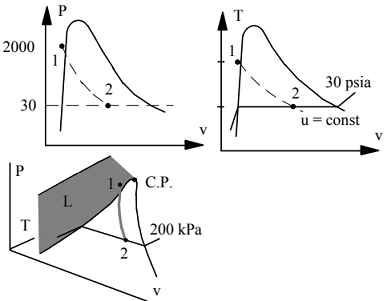
C.V.: Containment room and reactor.
Mass: m2 = m1 = Vreactor/v1 = 50/0.02172 = 2295.7 lbm
Energy m(u2 - u1) = 1Q2 - 1W2 = 0
⇒ u2 = u1 = 552.5 Btu/lbm
State 2: 30 lbf/in.2, u2 < ug ⇒ 2 phase
u = 552.5 = 218.48 + x2 869.41
⇒ x2 = 0.3842
v2 = 0.017 + 0.3842 x 13.808 = 5.322 ft3/lbm
V2 = mv2 = 2295.7 x 5.322 = 12218 ft3