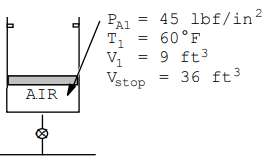
A mass-loaded piston/cylinder containing air is at 45 lbf/in.2, 60 F with a volume of 9 ft3, while at the stops V = 36 ft3. An air line, 75 lbf/in.2, 1100 R, is connected by a valve, The valve is then opened until a final inside pressure of 60 lbf/in.2 is reached, at which point T=630 R. Find the air mass that enters, the work, and heat transfer.