qH = 250 kJ/kg , TH = 600 K, TL = 300 K, P3 = 75 kPa
The states as
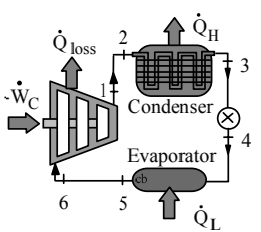
1 : 600 K , 2: 600 K, 3: 75 kPa, 300 K 4: 300 K
Since this is a Carnot cycle and we know the temperatures the efficiency is
η = 1 − TL/TH = 1 - 300/600 = 0.5
and the net work becomes
wNET = ηqH = 0.5 × 250
= 125 kJ/kg
The heat rejected is
qL = qH – wNET = 125 kJ/kg
After heat rejection
qL = RTL ln (v3/v4)
v3 = RT3 / P3 = 0.287 x 300 / 75 = 1.148 m3/kg
v4 = v3 exp(-qL/RTL) = 1.148 exp(−125/0.287 × 300) = 0.2688 m3/kg
P4 = RT4 / v4 = 0.287 × 300 / 0.2688 = 320 kPa