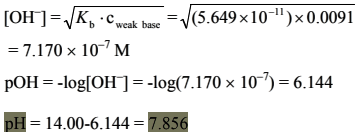(a) c(formic acid) = 0.010 M
Ka = 1.77 × 10–4
As we do not have the three orders of magnitude difference between the concentration and the acidic ionization constant, the more complicated formula has to be used for the calculation of the pH.
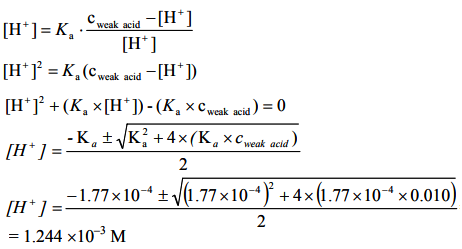
pH = -log[H+ ] = -log(1.244×10–3) = 2.905
(b) n(formic acid) = 100 × 0.010 = 1.00 mmol
n(NaOH) = 0.100 × 8 = 0.800 mmol
After the addition of 8.00 mL of NaOH solution, we get a basic buffer solution of HCOONa/HCOOH.
n(formic acid) = 0.200 mmol
n(sodium formiate) = 0.800 mmol
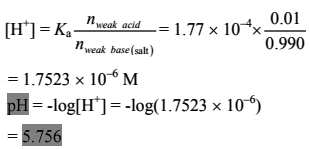
(c) n(formic acid) = 100 × 0.010 = 1.00 mmol
n(NaOH) = 0.100 × 9.90 = 0.990 mmol
After the addition of 9.90 mL of NaOH solution, we get a basic buffer solution of HCOONa/HCOOH.
n(formic acid) = 0.010 mmol
n(sodium formiate) = 0.990 mmol
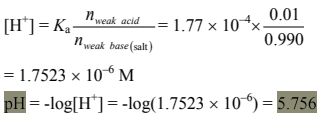
(d) n(formic acid) = 100 × 0.010 = 1.00 mmol
n(NaOH) = 0.100 × 10= 1.00 mmol
n(sodium formiate) = 1.00 mmol
The salt, sodium formiate is a weak base, so first the basic ionization constant should be calculated.

In this case cweak base >>> Kb, that is the equation to use is: