Given that:
Volume of vessel V = 5m3
Pressure of steam P = 100KPa
Volume of vapour Vg = 4.95m3
Volume of liquid Vf = 0.05m3
Since, the vessel is a closed container, so applying first law analysis, we have:
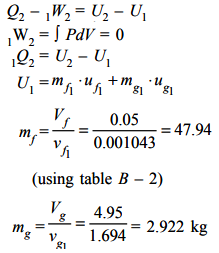
The final condition of the steam is dry and saturated but its mass *** the same. The specific volume at the end of heat transfer = vg2
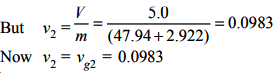
The pressure corresponding to vg= 0.0983 from saturated steam table is 2030kPa or 2.03 bar.
At 2.03 bar U2 = ug2 . m = (47.94 + 2922) × 2600.5 = 132.26 MJ
1Q2 = U2 – U1 = 132.26 – 27.33 = 104.93 MJ.