(i) ns2 np6
(ii) Zero
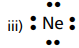
(iv) They are stable as they have 8 electrons (except Helium) in their outer most orbit.
Prepare a table that shows l, m values for n = 1, 2, 3, 4.
| n | l | ml |
| 1 | 0 | 0 |
| 2 | 0 | 0 |
| | 1 | – 1, 0, + 1 |
| 0 | 0 |
| 3 | 1 | – 1, 0, + 1 |
| 2 | – 2, – 1, 0, + 1, + 2 |
| 0 | 0 |
| 4 | 1 | – 1, 0, + 1 |
| 2 | – 2, – 1, 0, + 1, + 2 |
| 3 | – 3, – 2, – 1, 0, + 1, + 2, + 3 |