Correct Answer is: (D) Iodine monochloride – London dispersion force
All molecules have London dispersion force because of number of polarizable electrons. Non polar molecules have only London dispersion force because of polarisable electron. Benzene is non polar molecule. Polar molecules have dipole-dipole attraction in addition to London dispersion force.
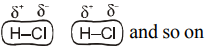
Molecules with, O–H, N–H, or F – H bond have hydrogen bonding in addition to London dispersion force.
Molecule ICl is polar, so in addition to London dispersion force it has dipole-dipole attraction also.