The electronic configuration of the central carbon atom (z = 6) in methane is 2, 4. Each of the four electrons present in the valence shell forms shared pairs with the electrons of the hydrogen atoms as shown below:
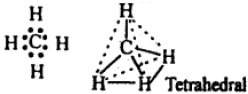
Methane has a tetrahedral structure which is multiplanar, in which carbon atom **** at the centre and the four hydrogen atoms lie at the four comers of regular tetrahedron. All H – C – H bond angles are 109.5°