When methyl bromide undergoes hydrolysis with aqueous potassium hydroxide, methyl alcohol is formed.
CH3 – Br + KOH → CH3 – OH + KBr
This mechanism involves only one step.
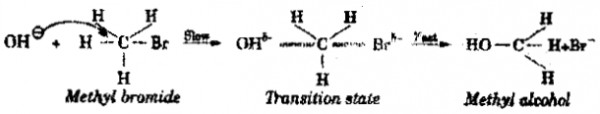
The nucleophile OH–** from the rear side of the leaving group. A transition state with partial formation of C-OH bond and partial breaking of C-Br bond takes place simultaneously. The rate of the reaction depends both on the concentration of nucleophile as well as alkyl halide. Hence, it is a second order reaction. In SN2 reaction, complete inversion of configuration takes place.