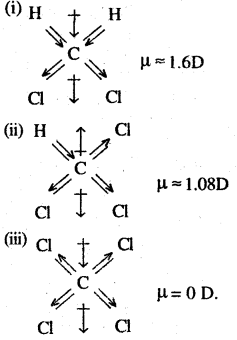
CCl4 is a symmetrical molecule. Therefore, the dipole moments of all four C-Cl bonds cancel each other. Hence its resultant dipole moment is zero.
As shown in the above figure, in CHCl3, the resultant dipole moments of two C-Cl bonds is opposed by the resultant dipole moments of one C-H and one C-Cl bond. Since the resultant of one C-H and one C-Cl bond is smaller than the resultant of the two C-Cl bonds dipole moments, the opposition is to a small extent. As a result CHCl3 has a small net dipole moment.
On the other hand, in case of CH2,Cl2 the resultant of the dipole moments of two C-Cl bonds is strengthened by the resultant of the dipole moments of two C-H bonds. As a result, CH2Cl2 has a higher dipole moment. Hence CH2Cl2 has the highest dipole moments among the three compounds.
Hence, the given compounds can be arranged in the increasing order of their dipole moments as
CCl4 < CHCl3 < CH2Cl2