The average atomic mass of a sample X= 16.2 u.
Let the % of isotope of (_{16}^8H) be y and % of isotope of y = (100 – y)%
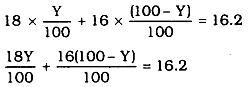
fi 18y + 1600 = 16y = 1620
fi 2y = 1620 – 1600
∴ 2y = 20
y = 10
Percentage of isotope X = 10%
Percentage of isotope y = 100 - 10 = 90%.