For non-volatile substance, relative lowering in vapour pressure of solution is equal to the mole faction of solute.
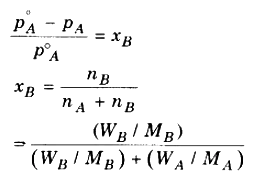
Where,
nB = Number of of solute
nA = Number of of solvent
WB = Mass of solute
WA = Mass of solvent
MB = Molecular mass of solute
MA = Molecular mass of solvent
According to. Raoult’* law
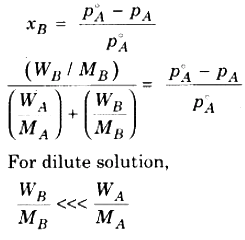
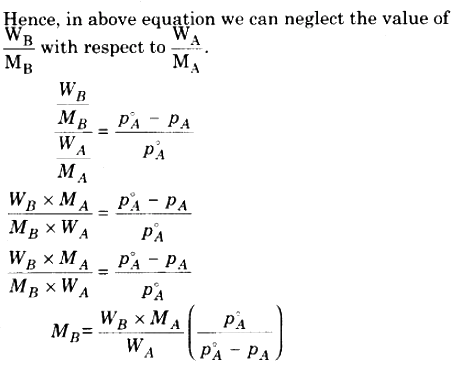
With the help of above equation we can calculate the value of molecular mass of non volatile solute, if all other factors in equation are known.