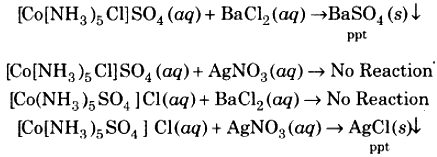Ionisation isomerism:- Ionisation isomerism occurs when same molecular formula gives different ions in the solution. For example, [Pt(NH3)4Cl2]Br2 and [Pt(NH3)4Br2]Cl2 are ionisation isomers.
[CO(NH3)4Cl]SO4 – IUPAC
Name – Pentaammine chloridocobalt (III) sulphate
[CO(NH3)5SO4]Cl – IUPAC
Name – Pentaammine sulphato cobalt (III) chloride
On dissolving in water, the two compounds will give different ions in the solution which can be tested by adding AgNO3 solution and BaCl2 solution. When Cl– ions are the counter ions, a white ppt. will be obtained with the AgNO3 and BaCl2 solution. If SO2-4 ions are the counter ions, a white ppt is obtained. It is shown by the following equations: