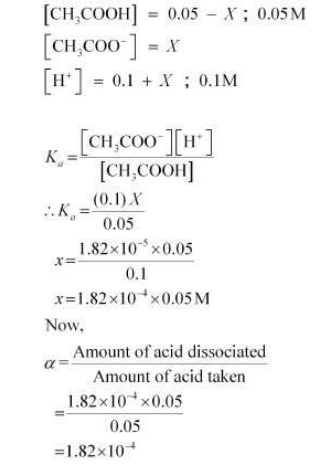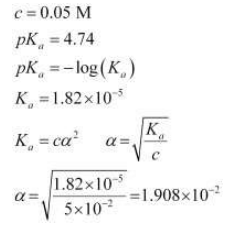
When HCl is added to the solution, the concentration of H+ ions will increase. Therefore, the equilibrium will shift in the backward direction i.e., dissociation of acetic acid will decrease.
Case I: When 0.01 M HCl is taken.
Let x be the amount of acetic acid dissociated after the addition of HCl.
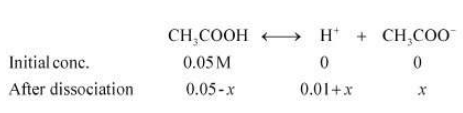
As the dissociation of a very small amount of acetic acid will take place, the values i.e., 0.05 – x and 0.01 + x can be taken as 0.05 and 0.01 respectively.

Case II: When 0.1 M HCl is taken.
Let the amount of acetic acid dissociated in this case be X. As we have d