Let the maximum concentration of each solution be x mol/L. After mixing, the volume of the concentrations of each solution will be reduced to half i.e., x/2 .
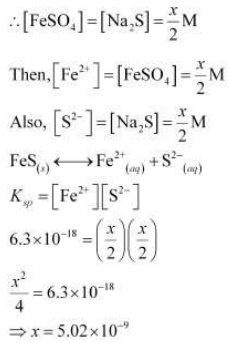
If the concentrations of both solutions are equal to or less than 5.02 × 10–9 M, then there will be no precipitation of iron sulphide.