Answer: We have
(i) AgNO3 ionizes in aqueous solutions to form Ag+ and NO3- ions.
On electrolysis, either Ag+ ions or H2O molecules can be reduced at the cathode. But the reduction potential of Ag+ ions is higher than that of H2O.
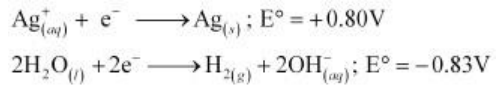
Hence, Ag+ ions are reduced at the cathode. Similarly, Ag metal or H2O molecules can be oxidized at the anode. But the oxidation potential of Ag is higher than that of H2O molecules.
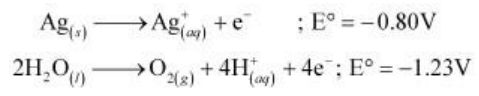
Therefore, Ag metal gets oxidized at the anode.
(ii) Pt cannot be oxidized easily. Hence, at the anode, oxidation of water occurs to liberate O2. At the cathode, Ag+ ions are reduced and get deposited.
(iii) H2SO4 ionizes in aqueous solutions to give H+ and SO42- ions.
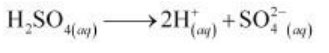
On electrolysis, either of H+ ions or H2O molecules can get reduced at the cathode. But the reduction potential of H+ ions is higher than that of H2O molecules.
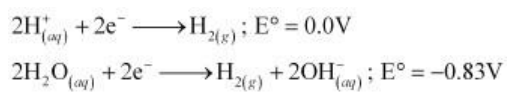
Hence, at the cathode, H+ ions are reduced to liberate H2 gas.