Addition of HBr to propene is an example of an electrophilic substitution reaction. Hydrogen bromide provides an electrophile, H+.This electrophile attacks the double bond to form 1° and 2° carbocations as shown:
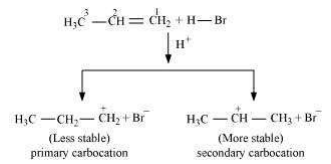
Secondary carbocations are more stable than primary carbocations. Hence, the former predominates since it will form at a faster rate. Thus, in the next step, Br– attacks the carbocation to form 2 – bromopropane as the major product.
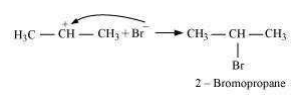
This reaction follows Markovnikov’ rule where the negative part of the addendum is attached to the carbon atom having a lesser number of hydrogen atoms. In the presence of benzoyl peroxide, an addition reaction takes place anti to Markovnikov’ rule. The reaction follows a free *** chain mechanism as:
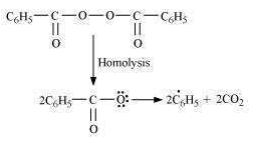
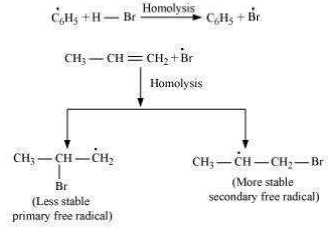
Secondary free **** are more stable than primary ****. Hence, the former predominates since it forms at a faster rate. Thus, 1 – bromopropane is obtained as the major product.
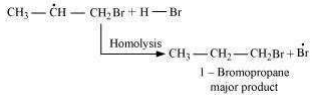
In the presence of peroxide, Br free *** acts as an electrophile. Hence, two different products are obtained on addition of HBr to propene in the absence and presence of peroxide.