The reaction of hydrogen and iodine monochloride is given as:
H2(g) + 2ICl(g) → 2HCl(g) + I2(*)
This reaction is of first order with respect to H2(g) and ICl(g) following mechanisms were proposed.
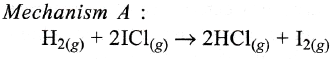
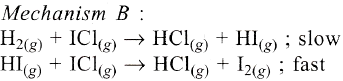
Which of the above mechanism(*) can be consistent with the given information about the reaction?
(a) A and B both
(b) Neither A nor B
(c) A only
(d) B only