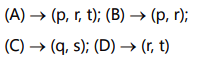Correct option A → p, r, t; B → p, r; C → *q, ; D → r, t**
Explanation
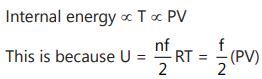
Here, n = number of *****, f = degree of freedom.
∴ If the product PV increases then internal energy will increase and if product decreases the internal energy will decrease.
Further, work is done on the gas, if volume of gas decreases. For heat exchange,
Q = W + Δ U
Work done is area under p–V graph. If volume increases work done by gas is positive and if volume decrease work done by gas is negative. Further ΔU is positive if product of PV is increasing and ΔU is negative if product of PV is decreasing.
If heat is taken by the gas, Q is positive and if heat is lost by the gas, Q is negative.
Keeping the above points in mind the answer to this question is as under